Milk is a familiar beverage in our daily lives. But have you ever wondered:
What are the main components of milk?
What are the structure and properties of these biological molecules?
How can we extract and identify these components?
In their recent Biology class, the young scientists of 9Cannes conducted exciting experiments to find the answers. In the previous lesson, they successfully extracted proteins, sugars, and minerals from milk. This time, the TDSers’ task was to analyze the physical and chemical properties of the collected samples. The class was divided into 4 groups, taking turns to carry out the experiments.



Observing molecular structures: Through the microscope, “Dewey explorers” stepped into the dynamic microscopic world. The structures of whey protein, casein, sugar, and more appeared vividly, revealing their complex and intricate forms. These details amazed the students as they observed the “shape” of molecules firsthand—something they had previously only seen in textbooks.

Testing for monosaccharides with benedict’s solution: After dissolving the sample and gently heating it in water, the young scientists excitedly observed the solution change from blue to orange-yellow due to the reduction reaction. This color change indicated the presence of monosaccharides (glucose, fructose) in the test sample.



Determining melting points: Using tools such as a heating stove and a thermometer, TDSers carefully observed and recorded the melting points of each sample. This process helped them better understand the unique physical properties of different biological molecules like sugars and proteins.
Testing the solubility of samples in deionized water, exploring the correlation between molecular structure and solubility.
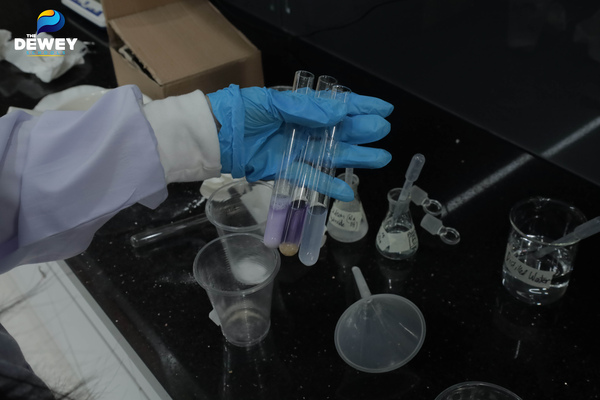
Hong Ngoc (9Cannes) enthusiastically shared: “Through this lesson, I’ve gained many fascinating insights. For example, sugar dissolves easily and quickly in water due to its polar nature, which allows it to interact well with water molecules. On the other hand, casein protein is less soluble in deionized water because of its larger and more complex structure.”



Through these experiments, students not only gained a deeper understanding of scientific concepts but also developed teamwork skills, mastered the use of laboratory equipment, and fostered a love for science. With this unique experiential learning approach, every lesson becomes an exciting scientific journey, sparking curiosity and a passion for exploration in each student.


























